
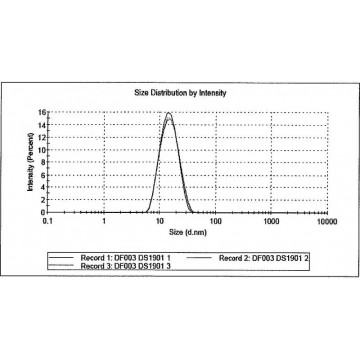
These results are contrasted with those obtained from serum albumin (BSA). This is demonstrated clearly with monoclonal antibody (mAb). Protein aggregates can easily reach large sizes, on the order of several hundred nm or larger, which complicate this already challenging measurement. Size determination of such small particles is by itself difficult as diffusion coefficient, which is the basis for the calculation of hydrodynamic size, is typically very high thus requiring a sophisticated high speed correlator with appropriately spaced delays for determination of the autocorrelation function for such rapidly diffusing particles. Many common globular proteins are very small in hydrodynamic size, with monomers rarely greater than 10 nm, and many approaching 1 nm or smaller on the low end. Proteins, however, still represent a challenge by themselves. Measurement of speed of movement, or translational diffusion, is achieved by determination of the autocorrelation function of the small fluctuations in intensity of light scattered by the sample at a given detection angle.ĭLS is a well know and powerful technique for the size determination of nanoparticles and proteins, its value has been demonstrated in innumerable publications. The speed of this Brownian motion is function of size large particles will show slow speed while smaller ones will move faster. The measurement is performed by directing a monochromatic beam of laser light through the sample in which the suspended particles undergo random thermal motion according to Brownian diffusion. The fundamental principle of DLS and all calculations following the measurement itself, is the determination of the translational diffusion coefficient of particles in suspension. However, in order to extract the information available from a DLS measurement, and most of all to assure the extracted values are representative for the given sample, we must take a step back and understand what DLS really measures and how, out of this primary information, final results of size and size distributions are calculated. As such, application of DLS is almost unlimited, ranging from polymer solutions, thus dissolved systems and sizes on the low nanometer range, up to particle dispersion with sizes ranging up to 10 µm. This is achieved without need of prior separation of any kind, mechanical or chemical. Best of all, DLS will supply, without the need of a calibration, not only size of the sample under investigation, but also, within certain limitations, size distribution information. Ease of use, fast availability of results within minutes and a relatively low sample volume requirements, define this technique as best and overcome any disadvantage we might want to consider. This principle is demonstrated with a pharmaceutically relevant protein, monoclonal antibody, mAb, studied in both its monomeric and aggregated states.ĭynamic Light Scattering (DLS) has become a major asset in the lab toolbox for the characterisation of colloids. In this scenario scattering from aggregated protein easily dominates the signal of free, or monomeric protein. Many common globular proteins are very small in hydrodynamic size, with monomers rarely greater than 10 nm.


 0 kommentar(er)
0 kommentar(er)
